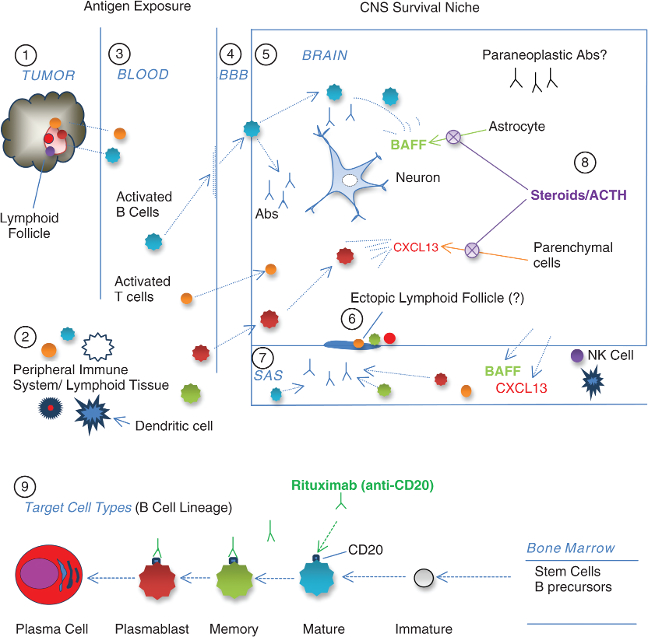
1 As part of immune defense against neuroblastoma, various mature immune cell types infiltrate the tumor lymphoid follicles (tumor-infiltrating lymphocytes or TILs).
2 The tumor instigates a cross-reactive autoimmune response that occurs in the secondary lymphoid organs of the peripheral immune system. Dendritic cells are the most potent stimulators of naïve T cells.
3 Under the prevailing theory, whether by molecular mimicry or other mechanisms, antigen-exposed blood-borne immune cells attack the brain and react to some unidentified onconeural antigen(s) under a unique set of circumstances, which may be influenced by genetics and the environment.
4 Expressed on the vascular endothelium, chemokines CXCL13 and CXCL10 attract activated B and T cells, memory B cells, and plasmablasts, which permeate the blood–brain barrier (BBB) early in OMS. B cells follow the chemotactic gradient into the brain. Unlike T cells, B cells recognize antigens in their native form, and most of their actions in autoimmune brain disorders are proinflammatory. The predilection of B1 cells, which predominate in toddlers, to the serous cavities (pleural and peritoneal) may be relevant to the body-cavity neuroblastomas.
5 A neurogenic survival niche is created by B-cell trophic factors, such as BAFF, a cytokine B-cell activating factor, and CXCL12, a plasma cell survival factor. The source of these nurturing cytokines may be the astrocyte or monocyte/macrophage. As a result, invading B cells that otherwise would die are able to survive, and antibodies (abs) are secreted locally. B cells may secrete other cytotoxic substances too.
6 CXCL13 may induce B cells to participate in lymphoneogenesis, along with T cells and plasma cells (speculation based on meningeal ectopic lymphoid follicles in multiple sclerosis).
7 In the subarachnoid space (SAS), cerebrospinal fluid (CSF) B-cell frequency increases, and the “expansion” is often accompanied by an inversion of the normal ratio of T1 helper/inducer (CD4+) to T2 cytotoxic/suppressor (CD8+) cells, suggesting immune dysregulation. Autoantibodies, detectable as OCB, are secreted by B cells against unidentified antigenic targets. Brain antigens also leak into the CSF.
8 Glucocorticoids and corticotropin reduce or normalize production of CXCL13 and BAFF as detected in the CSF. They may not be sufficient to stop autoantibody production.
9 Anti-CD20 monoclonal antibodies target only B-cell lineage cells that express CD20 for destruction; long-lasting plasma cells (“antibody factories”) escape, unless plasmablasts can be destroyed before they terminally differentiate. Clinical benefit has resulted from removal of pathogenic B lymphocytes with anti-B-cell monoclonal antibodies and abrogating B-cell survival factors by high-dose steroidogenic agents.